
Neuroethology
Lecture 29 - Flexibility
and adaptability in neural systems

How can neural systems change their behaviour?
One way is to use input to reconfigure network dynamics.

The lobster stomatogastric system
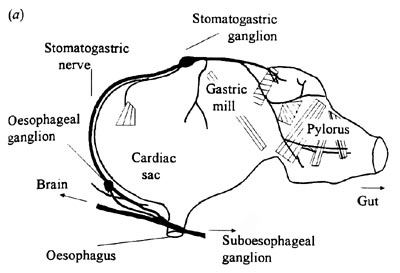
The infamous stomatogastric ganglion
STG consists of 30 neurons, almost all of which are motor neurons. (The anterior burster - AB - is the only neuron in the pyloric network that is not a motor neuron.) The networks of neurons in the STG produce two distinct rhythms, the gastric mill rhythm (period 5 - 10 secs) and the pyloric rhythm (period 0.5 - 2 secs).

The STG receives input from a pair of neurons called the pyloric suppressors (PS), which fire when food is sensed in the mouth. The PS neurons are electrically coupled, and so behave as a single functional unit. Input from PS switches the STG from producing one set of motor patterns to producing a second pattern; PS activity causes synchronous activity of the gastric mill and pyloric region.

Another way for a neural system to change its behaviour is to utilise stable states in the component neurons.
Many cells are not silent in the absence of external stimuli. The firing patterns of these cells are determined by membrane properties, such as the resting potential of the membrane, and the time course of different inwards and outwards currents. These properties (or parameters) can be modelled on a computer to describe the stable state of the neuron
Changing states in neurons(how to change the firing properties of the cell)
One way to change the firing properties of a cell is to change the membrane properties (parameters). This can be achieved using neuromodulators or neurohormones. The changes brought about in this way often display linear dynamics. This means that a certain change in neuromodulator level will produce a corresponding change in firing rate.

Some neurons, on the other hand, have more than one stable state for a given set of membrane properties (parameters): they exhibit multi-stable states. Switching between these states is parameter independent, meaning that you do not have to change the membrane properties of the cell to alter the firing state. The transition between states can be elicited by transient synaptic input. In some cases this transient input must occur at particular points in the firing cycle of the cell - in other words the transition is phase sensitive. Because the transition is abrupt, essentially an all-or-nothing response, multi stable neurons show non-linear dynamics.

Introducing the R15
neuron in our new friend Aplysia
Aplysia is a sea slug that has been studied extensively by neuroethologists
because it is hardy and has large, easily accessible neurons.
One such neuron is the interneuron R15. Computational models of
the R15 show multiple stable states (up to 8). These states can
be induced by brief perturbations of the membrane potential Recordings
from R15 showed (at least) 2 stable states: bursting and
beating. Transient perturbations of the membrane potential
can cause the cell to switch states, and serotonin modulates the
likelihood and duration of these state-changes.

This figure shows the effect of a brief electrical stimulation on the bursting mode of an R15 neuron. In the lower trace the neuron is switched from one state to another and back again.

This figure shows the neuromodulatory effect of serotonin, which increases the likelihood and duration of state changes. This is a nice example of combining parameter-dependent and parameter-independent changes in cell behaviour.
see review: Marder, E. Computational dynamics in rhythmic neural circuits. The Neuroscientist (1997) 3(5):295-302